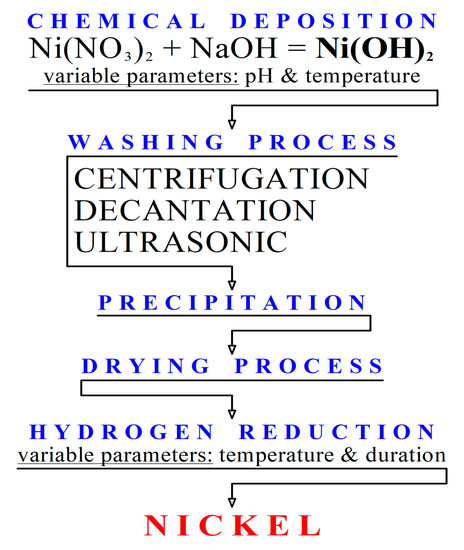
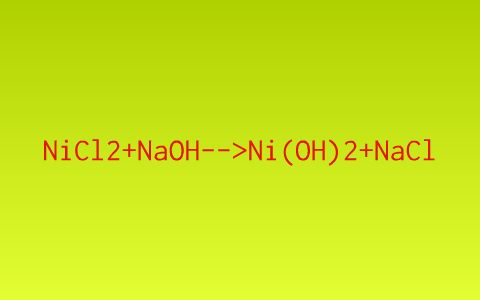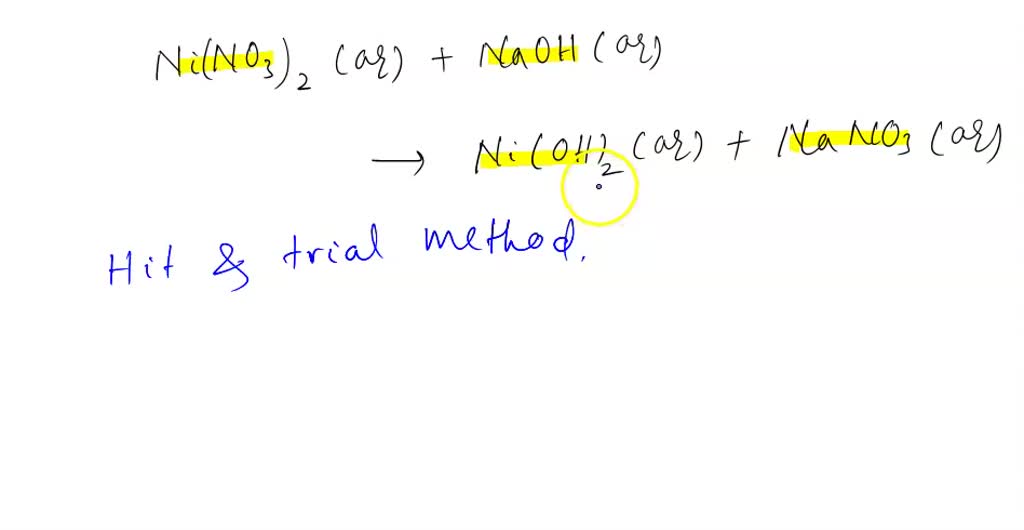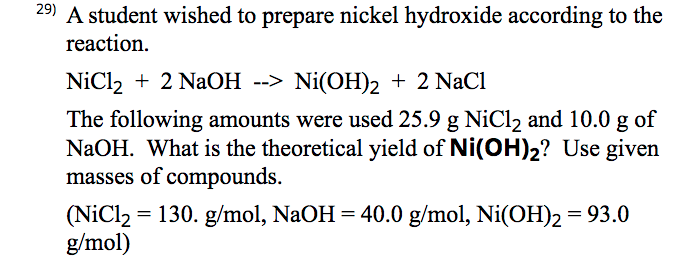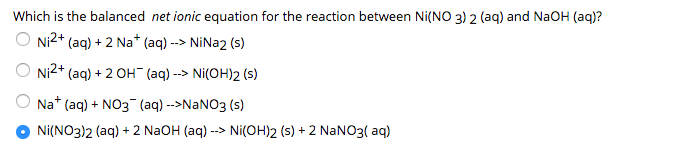Influence of NaOH, Ni/Al2O3 and Ni/SiO2 catalysts on hydrogen production from the subcritical water gasification of model food waste compounds - ScienceDirect

Metals | Free Full-Text | Resistivity and Passivity Characterization of Ni-Base Glassy Alloys in NaOH Media

Figure 5 from Thermodynamic model of Ni(II) solubility, hydrolysis and complex formation with ISA | Semantic Scholar

Strongly Coupled Ni/Ni(OH)2 Hybrid Nanocomposites as Highly Active Bifunctional Electrocatalysts for Overall Water Splitting | ACS Sustainable Chemistry & Engineering

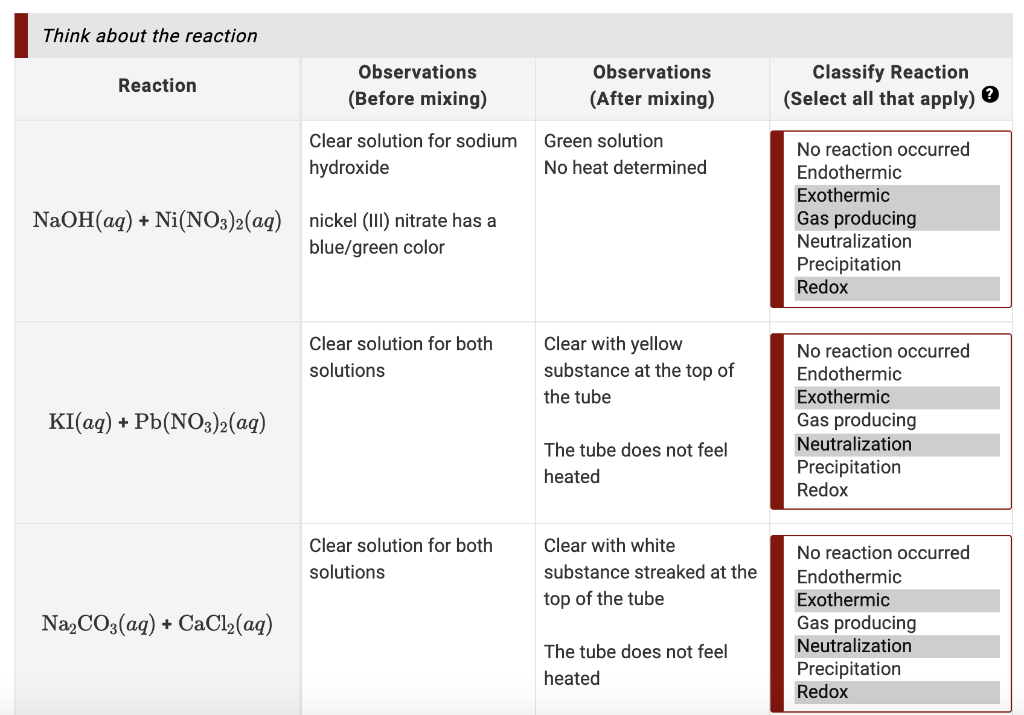
Chapter 4: Aqueous Reactions Solution: Solvent: substance present in the larger amount Solute: substance(s) dissolved in solvent, generally present in. - ppt download

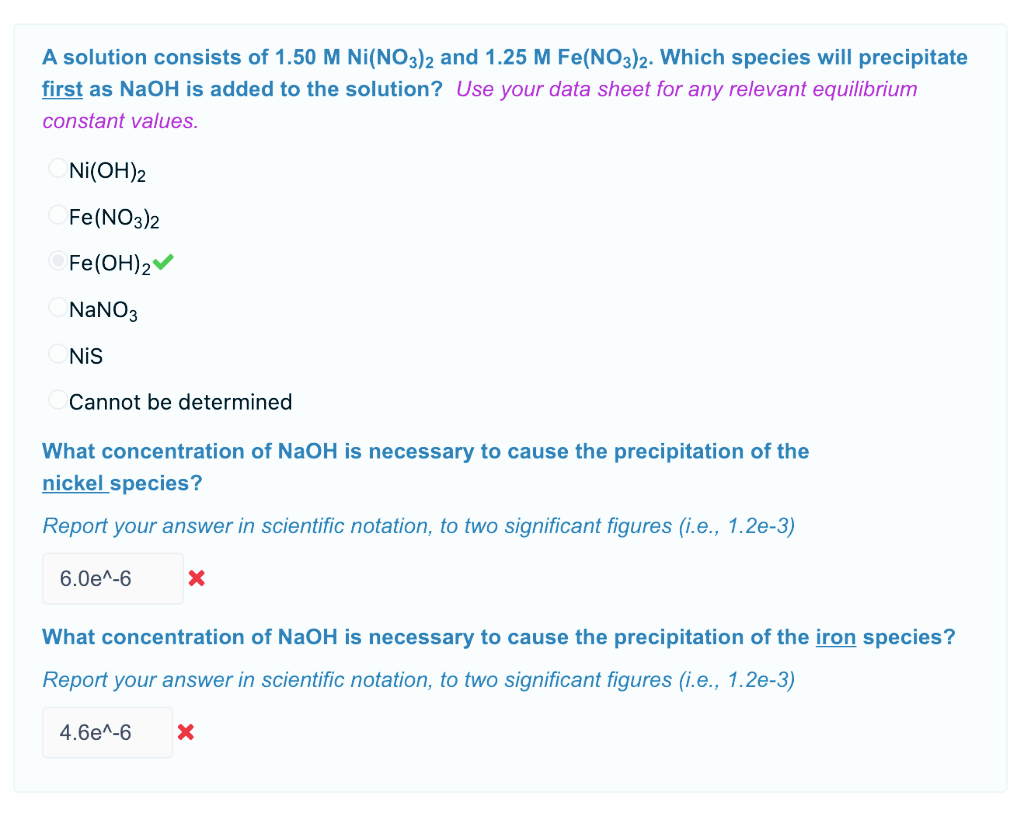
Calculate the molar solubility of Ni(OH)2 in 0.10M NaOH. The ionic product of Ni(OH)2 is..... - YouTube

a CV profiles of a Ni(poly) electrode obtained in 0.10 M aqueous NaOH... | Download Scientific Diagram
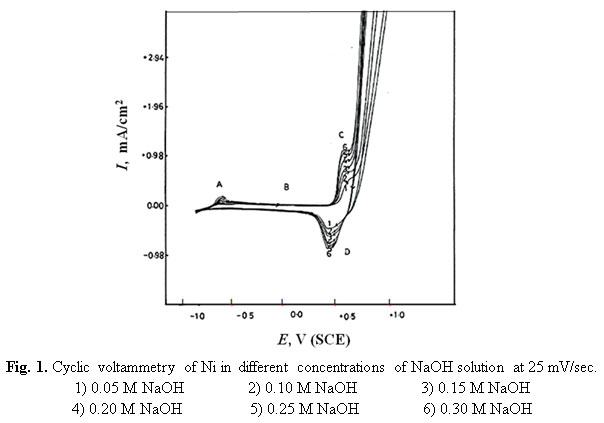
Corrosion Performance of Stainless Steel and Nickel Alloys in Aqueous Sodium Hydroxide as Revealed from Cyclic Voltammetry and Potentiodynamic Anodic Polarization : Oriental Journal of Chemistry
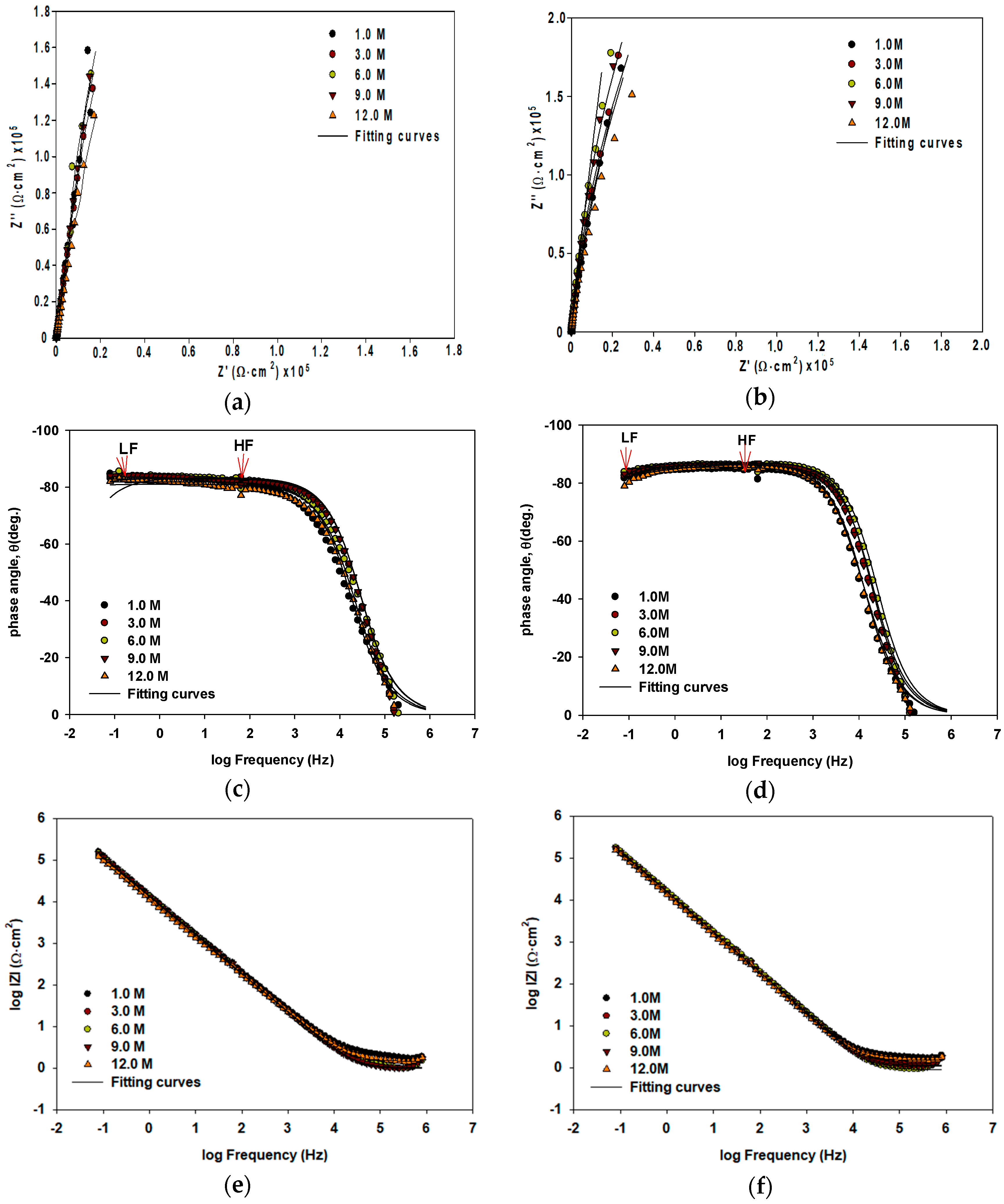
Metals | Free Full-Text | Resistivity and Passivity Characterization of Ni-Base Glassy Alloys in NaOH Media

Find out the solubility of Ni(OH)2 in 0.1 M NaOH.Given that the ionic product of Ni(OH)2 is 2×10-15 - YouTube

NEET 2020 SOLUTION -Find out the solubility of Ni(OH)2 in 0.1 M NaOH . Given that the ionic product - YouTube